Speciality Polymers are the class of some High Performance Polymers (HPP), including plastics, polymers, fluids, membranes, smart hydrogels and elastomers that are designed to meet the critical requirements that engineers face every day in key industries including, Plastics, Automobiles, Aeronautics, Smart Devices, Healthcare, Pharmacy, Energy Production and Storage. This article is a short but diversified study of Post Grad. Students in the area of speciality polymers, their types and applications in engineering, technology, life sciences and research. It includes diverse applications of Ionic Polymer Metal Composites (IPMC), Liquid Crystal Polymers (LCPs), Synthetic Polymer Membranes, Smart Hydrogels, and Dendritic Polymers.
......................................................................................................................................................
Polyimides (PI)
Polyimides (PI) are high-performance polymers of imide monomers
which contain two acyl groups (C=O) bonded to nitrogen (N). These
polymers are known for their high temperature performance in the
400-500°C range as well as chemical resistance.
They are used to replace the conventional use of glass, metals and even steel in many industrial applications.
Polyimides offer excellent mechanical properties and thus find use in applications that demand rugged organic materials, e.g.
- High temperature fuel cells
- Flat panel display
- Aerospace applications
- Chemical and environmental industries
- As well as various military applications
They are available for use as plastics, films, laminating resins, insulating coatings and high temperature structural adhesives.
Polyimides exist in two formats: thermosetting and thermoplastic.
Depending upon the constitution of their main chain, Polyimide can be classified as aliphatic, aromatics, semi-aromatics thermoplastics and thermosets.
- Aromatic polyimides are derived from an aromatic dianhydride and diamine.
- Semi-aromatic ones contain any one of the monomer aromatics: i.e., either the dianhydride or diamine is aromatic, and the other part is aliphatic.
- Aliphatic polyimides consist of the polymers formed as a result of the combination of aliphatic dianhydride and diamine.


Polyimides have been in mass production since 1955.
» View All Commercially Available PI Grades & Suppliers in Omnexus Plastics Database
This plastic database is available to all, free of charge. You can filter down your options by property (mechanical, electrical…), applications, conversion mode and many more dimensions.
Continue reading or click to go on specific section of the page:
- Synthesis of Polyimides
- Well Known Properties of Polyimides
- Strengths and Limitations of Polyimides
- Polyimides (PI) - Popular Applications
- How to Process PI?
Synthesis of Polyimides
Polyimides are prepared by incorporating highly stable and rigid heterocyclic ring systems into the polymer chain. Hence, the presence of an inert imide ring and high interchain interaction, i.e., high cohesion among the polymer chains imparts high thermal stability in the polymer.
The classical method of polyimide synthesis is by reaction of a dianhydride and a diamine.
Synthesis of aromatic polyimides was first reported in 1908. However, due to the lack of processability via melt polymerization, significant advances in polyimide synthesis and processing were not realized. In early 1960s, DuPont was the first company to produce polyimide commercially. It was based on pyromellitic dianhydride and 4,4’diaminodiphenyl ether.

This type of reaction consisted of two steps
- The solution polycondensation of an aromatic diamine and a dianhydride to form poly(amic acid)
- Poly(amic acid) could be processed into a useful shape, followed by cyclodehydration of the amide-acid to form polyimide
The thermal stability of polymer can further be improved by incorporating aromatic rings on the backbone and/or side groups. In addition to such high thermal stability, the nature of the chemical structure consisting of rigid imide and aromatic rings always provides:
- Excellent mechanical toughness
- Excellent dielectric properties
-
High chemical resistance
- Photo reactivity
- Molecular recognition ability
- Nonlinear optical responsibility
- Light emitting ability, and so on
Well Known Properties of Polyimides
Polyimides exhibit an exceptional combination of thermal stability (>500 °C), mechanical toughness, and chemical resistance. They have excellent dielectric properties.- Additionally, the morphology of long, linear ordered chains provides solvent resistance to the aromatic polyimides.
The comparatively rigid structure of polyimides provides high glass transition temperature (Tg > 300°C) and imparts good mechanical strength and high modulus.
Polyimides (PI) - Popular Applications
Polyimide (also known as PI or Kapton) is widely employed for electronic applications in aerospace and automotive industry due to its excellent mechanical strength, electrically insulating properties and thermal stability.

Polyimide applications in electronics
mainly consist of wafer carriers and guides, test holders, chip trays,
hard disk drive components, electrical connectors, coil bobbins, wire
insulators and digital copier and printer components for the electronics
market.

Polyimide films are widely used as a dielectric substrate in flexible solar cells for their high thermal stability, toughness and flexibility. PI films are used to produce solar cells with maximum efficiency and yields and as substrates in thin film a-Si and CIGS photovoltaic applications due to its high thermal stability.
--------------------------------------------------------------------------------------------------------------
Polyolefin
A polyolefin is a type of polymer produced from a simple olefin (also called an alkene with the general formula CnH2n) as a monomer. For example, polyethylene is the polyolefin produced by polymerizing the olefin ethylene. Polypropylene is another common polyolefin which is made from the olefin propylene.
Polyolefins polymers are some of the most prevalent plastics used today and come in various types
- Polyethylene (PE) with subgroups
- high-density HDPE
- low-density LDPE
- linear low-density LLDPE
- Polypropylene (PP)
- Ehylene propylene diene monomer (EPDM) rubber
| Polymer Type | Symbol | Examples of use |
|---|---|---|
| HDPE | ♴ | fuel tanks, bottle caps, plastic bottles,… |
| LDPE | ♶ | liquid containers, tubing, plastic wrap,… |
| PP | ♷ | piping, carpet, roofing, hinges, auto parts,… |
| EPDM | seals, electrical insulation, roofing,… |
HDPE, LLDPE and LDPE are the highest volume grades.
Properties
- Polyolefin properties range from liquidlike to rigid solids, and are primarily determined by their molecular weight and degree of crystallinity.
- Polyolefin degrees of crystallinity range from 0% (liquidlike) to 60% or higher (rigid plastics).
- Crystallinity is primarily governed by the lengths of polymer's crystallizable sequences established during polymerization.
- Examples include adding a small percentage of comonomer like 1-hexene or 1-octene during the polymerization of ethylene, or occasional irregular insertions ("stereo" or "regio" defects) during the polymerization of isotactic propylene. The polymer's ability to crystallize to high degrees decreases with increasing content of defects.
- Low degrees of crystallinity (0–20%) are associated with liquidlike-to-elastomeric properties. Intermediate degrees of crystallinity (20–50%) are associated with ductile thermoplastics, and degrees of crystallity over 50% are associated with rigid and sometimes brittle plastics.
- Polyolefin surfaces are not effectively joined together by solvent welding because they have excellent chemical resistance and are unaffected by common solvents. They can be adhesively bonded after surface treatment (they inherently have very low surface energies and don't wet-out well (the process of being covered and filled with resin)), and by some superglues (cyanoacrylates) and reactive (meth)acrylate glues.
Ionic polymers/Ionomer
Ionic polymers are polymers, either organic or inorganic, which contain both covalent and ionic bonds in their molecular structure. This is the basic characteristic feature that distinguishes the ionic polymers from their conventional counterparts.
An ionomer (/ˌaɪˈɑːnəmər/) (iono- + -mer) is a polymer composed of repeat units of both electrically neutral repeating units and ionized units covalently bonded to the polymer backbone as pendant group moieties (side group). Usually no more than 15 mole percent are ionized. The ionized units are often carboxylic acid groups.
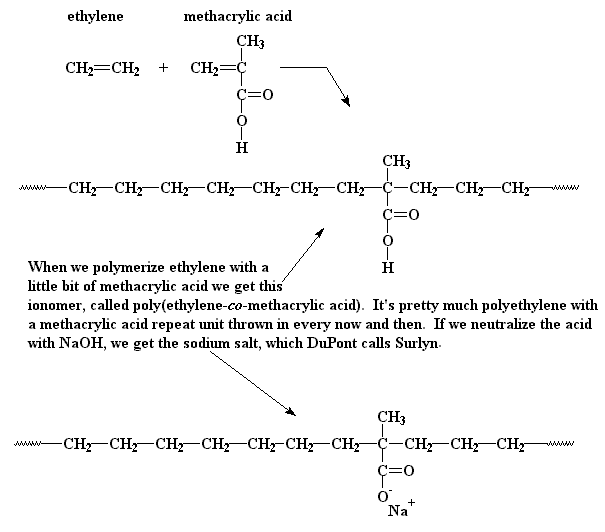
Ionomers using poly(ethylene-methacrylic acid) chains can also be used in film packaging due to their transparency, toughness, flexibility, resistance to staining, high gas permeability, and low sealing temperature. These qualities also translate to a high demand for using the ionomers in food-packing materials.
Some applications where ionomers were used to increase the toughness of the overall system include coatings, adhesives, impact modification, and thermoplastics, one of the most known examples being the use of Surlyn in the outer layer of golf balls.
The ionomer coating improves the toughness, aerodynamics, and durability of the golf balls, increasing their lifetime.
Ionomers can also be blended with resins to increase the cohesive strength without diminishing the overall tackiness of the resin, creating pressure sensitive adhesives for a variety of applications, including water or solvent-based adhesives.
Ionomers using poly(ethylene-methacrylic acid) chains can also be used in film packaging due to their transparency, toughness, flexibility, resistance to staining, high gas permeability, and low sealing temperature.
These qualities also translate to a high demand for using the ionomers in food-packing materials.
Glass ionomer cement is primarily used in the prevention of dental caries. This dental material
has good adhesive bond properties to tooth structure, allowing it to
form a tight seal between the internal structures of the tooth and the
surrounding environment.
------------------------------------------------------
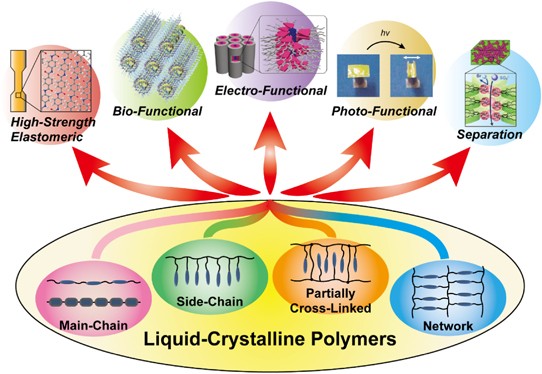
Liquid crystalline polymers (LCPs) are a special type of
thermoplastics that exhibit properties between highly ordered solid
crystalline materials and amorphous disordered liquids over a well
defined temperature range.
To date, thousands of LC polymers have
been synthesized. However, only a small number have become
commercially important LC materials. The three most common LCPs are
semi-aromatic copolyesters, copolyamides, and polyester-co-amides.
These polymers contain rigid rod-like or plate-like repeat units
with a high length-to-width ratio, the so called mesogenic groups,
that are able to self-assemble into anisotropic liquid crystals
(mesophases) upon cooling or under the action of an external field.
Three very common LC mesophases are nematic, smectic A and
smectic C
(see below). Nematic mesophases show only unidimensional
orientational
order in the direction of the long (in rod-shaped) or short (in
disc-shaped) molecular axes.
This is typically the flow direction during processing of the LC
resin. Smectic mesophases, on the other hand, show two-dimensional
orientational order. These LC’s have a lot in common with crystalline
polymers where molecules are arranged in layers (lamellae).
The long axes of the (rod-like) molecules are either perpendicular to
the plane (smectic A) or
it is inclined at an angle (smectic C).
Examples of Common Mesophases
The most common LCPs are liquid crystalline polyesters which can be produced by polycondensation of an aromatic hydroxycarboxylic acid and an aromatic dicarboxylic acid with an alipahtic diol as a coreactant. The aromatic blocks form liquid crystals in the copolymer while the aliphatic blocks act as flexible spacers. Several other flexible (hydrophobic) spacers such as alipahtic ethers and organosilicons can be part of the LCP. The spacers or flexible linkers impart mobility to the rigid mesogenic groups1 to ease cooperative self-assembly.
Examples of Typical Mesogenic Units
The highest volume commercial LCPs are liquid crystalline polyesters. One of the cheapest is self-reinforcing polyester which can be produced by co-polycondensation of p-hydroxybenzoic acid and terephthalic acid and/or 4,4i-dihydroxydiphenyl with ethylene glycol as a coreactant. Important manufacturers of this type of LCP are Celanese and Solvay.
LC polymers are also widely used as functional materials in all kinds of optic and optoelectronic devices because their anisotropic material properties such as refractive index, birefringence, selective light reflection and transmitance, color characteristics are tunable by temperature, mechanical stress, and electromagnetic radiation and fields. Important applications include temperature-sensing, data storage, display technology, telecomunication and numerous other optic and optoelectronic products.

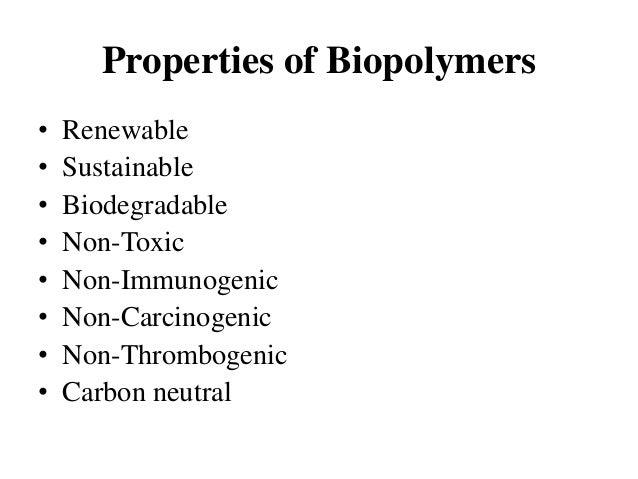
Biopolymers
- Biopolymers are natural polymers produced by the cells of living organisms.
- Biopolymers consist of monomeric units that are covalently bonded to form larger molecules.
- There are three main classes of biopolymers, classified according to the monomers used and the structure of the biopolymer formed: polynucleotides, polypeptides, and polysaccharides.
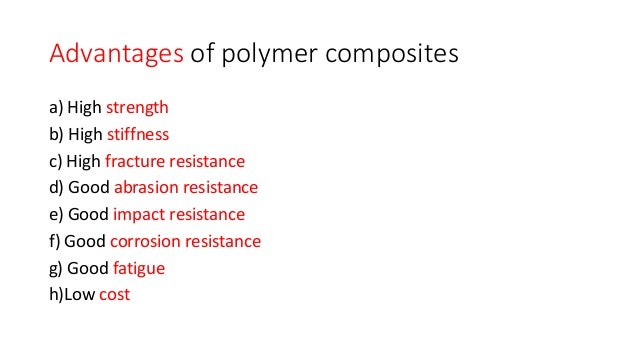
Polymer Composites
A polymer composite is a multi-phase material in which reinforcing fillers are integrated with a polymer matrix, resulting in synergistic mechanical properties that cannot be achieved from either component alone
What is a PMC used for?
Applications for PMCs include:
Automotive industry - Body panels, leaf springs, driveshaft, bumpers, doors, racing car bodies, and so on.
Aircraft and aerospace industry - Used in the construction of structural parts for military aircraft, space shuttles, and satellite systems. The main purposes of using PMCs are to reduce aircraft weight, which can improve its performance, and to reduce its costs.
Marine - Fibreglass boat bodies, as well as canoes and kayaks.
Sports goods - Used in performance footwear, sports equipment and other sporting goods because of their lightweight and high-strength properties.
Biomedical applications - Medical implants, orthopaedic devices, MRI scanners, X-ray tables, and prosthetics.
Electrical - Panels, housing, switchgear, insulators, and connectors. It also covers electronic devices like capacitors, Li-ion and flexible batteries and covers for digital portable equipment like headphones, etc.
Protective equipment - Since polymer matrix composites can withstand extreme hot or cold and other hazardous conditions, they are often made as raw materials for bulletproof vests and other armour.
Industrial - Chemical storage tanks, pressure vessels, pump housing, and valves. PMCs are also used in impellers, blades, blower and pump housings, and motor covers.
Structural - Polymer matrix composites are used to repair bridges and other construction materials and equipment like booms and cranes.
polymer electrolyte
A polymer electrolyte is also referred to as a solid solvent that possesses ion transport properties similar to that of the common liquid ionic solution. It usually comprises a polymer matrix and electrolyte, wherein the electrolyte such as a lithium salt dissolves in a polymer matrix.
Hybrid polymer electrolytes:
Hybrid polymer electrolytes represent one of the most versatile classes of solid polymer electrolyte. To a certain extent, these hybrid materials combine the most important properties of their constituents, such as high transparency (glass-like), low processing temperatures (polymer-like), sufficient thermal stability (silicone-like), with high performance yield and properties not found in either material individually. These materials display a number of advantages over simple salt-in-polymer electrolytes and are believed to be promising materials for application in secondary lithium batteries and electrochromic windows.
Dry polymer electrolyte
The dry polymer electrolyte generally contains an alkali metal salt complexed with the polymer matrix. The very first example of ‘dry solid’ polymer electrolyte is the poly(ethylene oxide) (PEO)-based system that showed very low ambient temperature conductivities on the order of 10−8 S cm−1. This system does not possess any organic liquid and thus the polymer host is used as a solid solvent. However, the cycling performance of this dry solid polymer electrolyte with lithium metal electrodes was not satisfactory as the usage was as low as 200–300 cycles.
Gel polymer electrolytes
Gel polymer electrolytes (GPEs) consist of a polymeric membrane onto which a minimum amount of classic “salt–solvent” combination is added. The salt and organic solvent can be picked from the list of common Li/Na salts and carbonate solvents used in liquid electrolytes. The solvent acts as a plasticizer and its addition results in the swelling of the polymer matrix, which physical aspect changes from a solid to a gel. The gellification of the membrane facilitates the interfacial contact with the electrodes in comparison to SPEs. The most common polymers used to prepare GPEs are polyacrylonitrile (PAN), polymethylmethacrylate (PMMA), polyvinylchloride (PVC), and polyvinylidene fluoride (PVDF);

sd
ReplyDelete