- Polymer degradation includes all changes in both the chemical structure and physical properties of polymers or polymer-based products that lead to the loss of properties such as tensile strength, color, shape, etc., under the influence of processing conditions, or one or more environmental factors (e.g., heat, light, or exposure to chemicals)
- Such loss of properties can occur by the breakage of polymer molecules, or by polymer fragmentation into pieces that may be small enough to disappear but which are still similar to the original material.
- The loss of properties in a finished product is undesirable and needs to be prevented or delayed, but in other cases the loss of properties at a given rate is desirable as it happens in the production of biodegradable polymers.
- The processes by which polymers can suffer degradation are thermal, mechanical, hydrolitic, chemical, biological, photolitic, ultrasonication, pollution contact, radiolytic, and sludge activation. The amount of time to have a certain degree of degradation depends on the type of polymer, morphology, molecular size, and the conditions to which it is subjected.
- Degradable plastics can be obtained using biopolymers (polymers derived from renewable biomass sources), such as poly(alkyl hydroxyalkanoates), poly(lactic acid), cellulose, and starch, among others.
- Synthetic degradable polymers, such as polycaprolactone
and poly(vinyl alcohol), can also be used to obtain degradable
plastics. These can be degraded by abiotic (hydrolysis, photolysis or oxidisation) and biodegradation mechanisms.
- Polymer abiotic degradation occurs by hydrolysis where the first step is the random hydrolytic cleavage of functional groups susceptible to be hydrolyzed without weight loss: for instance, ester and urethane groups. The second stage of degradation is when besides chain cleavage, there is weight loss.
- Polymer biodegradation happens when a polymeric material is broken down by microorganisms (bacteria, fungi, algae) into natural elements such as water and carbon dioxide.
- Degradable plastics can be classified as:
- (1) Environmentally biodegradable biopolymers (films, packaging, mulch) that should have no degradation or a low degree of degradation when in use and accelerated degradation when in the disposal stage; and
- (2) Biodegradable biopolymers which need to undergo controlled degradation when in application and which are mainly used in the medical sector (e.g., sutures, implants, and drug delivery systems).
- Degradation of biodegradable materials depends on a variety of factors including chemical structure of the polymer, morphology, processing conditions, form and size of the article, as well as environmental conditions, among other aspects.
Mechanism of polymer degradation
- The major factors causing degradation are heat, mechanical energy, radiation and ozone leading to various types such as thermal, thermo oxidative, photo degradation, chemical, biological, hydrolytic and degradation by irradiation.
- In a depolymerisation reaction, a reversal of polymerisation occurs which includes Initiation at chain ends, Depropogation, Termination.
- Depolymerization: In depolymerisation process low activity free radical takes place at the end of polymer chain.The change in molecular chain takes lot of time as the monomers are lost by polymeric chain one by one as per the mechanism of chain reaction.
- Random chain scission: Decrease in the molecular weight occurs rapidly as the backbone breaks down randomly which occurs at any site of backbone. Here two reactions namely disproportion termination and intermolecular chain transfer occurs resulting into formation of new free radicals having high reactivity.
- Side –group elimination: Bonds connecting the backbone are much stronger than the bonds which connect the side groups to the backbone chain. When depolymerisation occurs side groups are removed from the backbone before any further breakage.
Types of degradation:
Thermal degradation methods- The initiation of thermal degradation involves the loss of a hydrogen atom from the polymer chain as a result of energy input from heat or light. This creates a highly reactive and unstable polymer 'free radical' (R•) and a hydrogen atom with an unpaired electron (H•)
- Mechanical degradation
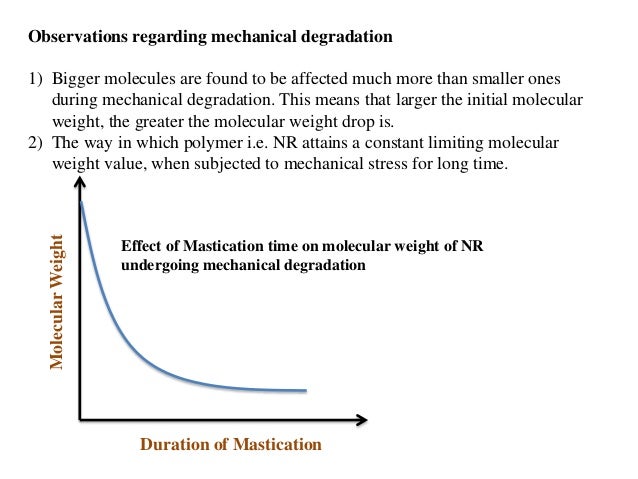
NR-Natural Rubber
- Biodegradation methods
Indeed, biodegradation is the process by which organic substances are
broken down into smaller compounds by living microbial organisms.
Biodegradable polymers are a special class of polymer that breaks down after its intended purpose by bacterial decomposition process to result in natural byproducts such as gases (CO2, N2), water, biomass, and inorganic salts.
These polymers are found both naturally and synthetically made, and largely consist of ester, amide, and ether
functional groups.
Photodegradation
- Photodegradation is degradation of a photodegradable molecule caused by the absorption of photons, particularly those wavelengths found in sunlight, such as infrared radiation, visible light, and ultraviolet light.
- However, other forms of electromagnetic radiation can cause photodegradation. Photodegradation includes photodissociation, the breakup of molecules into smaller pieces by photons.
- It also includes the change of a molecule's shape to make it irreversibly altered, such as the denaturing of proteins [breaking of many of the weak linkages, or bonds (e.g., hydrogen bonds)], and the addition of other atoms or molecules.
- A common photodegradation reaction is oxidation. Photodegradation in the environment is part of the process by which ambergris evolves from its fatty precursor. Photodegradation also destroys paintings and other artifacts.
- Light - induced polymer degradation, or photodegradation, includes the physical and chemical changes caused by irradiation of polymers with ultraviolet or visible light.
- In order to be effective, light must be absorbed by the substrate (polymeric system). Thus, the existence of chromophoric groups in the macromolecules is a prerequisite for the initiation of any photochemical reaction Schnabel.
- Ketones,
quinines, and peroxides are initiators for different reaction
degradation or chemical modification occurring in organic compounds.
They absorb light up to about 380 nm, which causes their excitation or
cleavage to radicals.
- Ultrasonic degradation
- Oxidative degradation involves the disintegration of macromolecules by the action of oxygen on the substrate (oxidation).
- Free radicals are formed which react with oxygen-producing oxy- and peroxy-radicals.
- These, on the other hand, can participate in many reactions: they may react with each other or remove hydrogen from polymer chains.
- Oxidative degradation can be initiated by three factors such as UV light, heat, or mechanical stress in the presence of oxygen atmosphere.
- Oxidative degradation can proceed according to two mechanisms: photooxidation and thermal oxidation.
- Photooxidation is caused by the action of UV light in the presence of oxygen, and due to its limited penetration capability, it takes place only on the surface and subsurface layers of polymer.
- Thermal oxidation can extend throughout the material. It takes place as a result of the simultaneous interaction of oxygen with the polymer and high temperature.
- Oxidation leads to formation of, hydroxyl, carbonyl, aldehyde groups, or peroxides, along the polymer chain or at its ends. As a result of oxidation, the mechanical properties and utility of the polymer are significantly reduced. This is due to secondary cross-linking and disruption of molecular chains, which may lead to softening of the surface of the polymer.
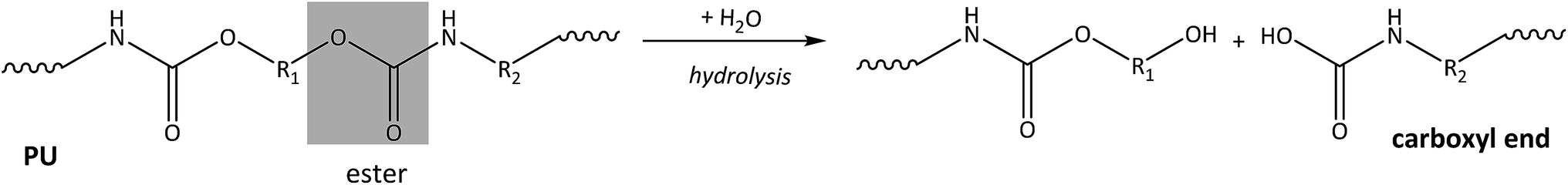
Hydrolytic degradations
No comments:
Post a Comment