Contents:
- Crystalline and amorphous states,
- melting and glass transition of polymer,
- factors affecting melting temperature (Tm) and glass transition temperature (Tg),
- detection and determination of Tg.
.................................................................................................................................................
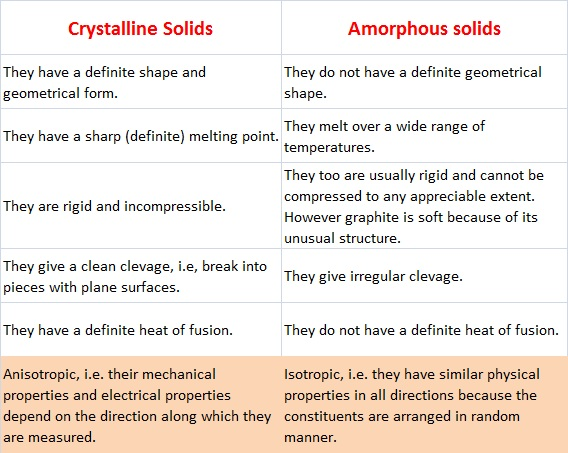
Crystallinity defines the degree of long-range order in a material, and strongly affects its properties.
The more crystalline a polymer, the more regularly aligned its chains.
Increasing the degree of crystallinity increases hardness and density of the polymer.
This is illustrated in poly(ethene). HDPE (high density poly(ethene)) is composed of linear chains with little branching. Molecules pack closely together, leading to a high degree of order. This makes it stiff and dense, and it is used for milk bottles and drainpipes.
The numerous short branches in LDPE (low density poly(ethene)) interfere with the close packing of molecules, so they cannot form an ordered structure. The lower density and stiffness make it suitable for use in films such as plastic carrier bags and food wrapping.
Linear low-density polyethylene (LLDPE) is a substantially linear polymer (polyethylene), with significant numbers of short branches, commonly made by copolymerization of ethylene with longer chain olefins (alkene).
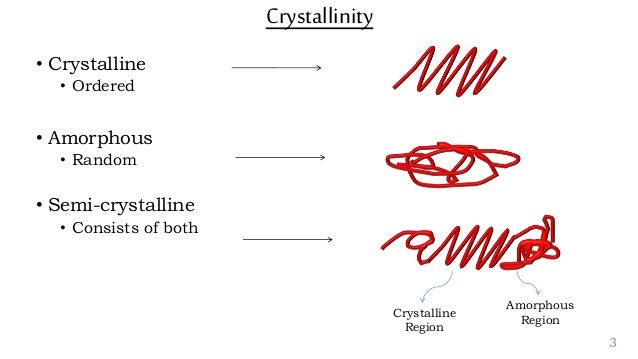
Often, polymers are semi-crystalline, existing somewhere on a scale between amorphous and crystalline.
This usually consists of small crystalline regions (crystallites) surrounded by regions of amorphous polymer.
Factors favouring crystallinity
In general, factors causing polymers to be more ordered and regular tend to lead to a higher degree of crystallinity.
- Fewer short branches – allowing molecules to pack closely together
- Higher degree of stereoregularity - syndiotactic and isotactic polymers are more ordered than atactic polymers.
- More regular copolymer configuration – having the same effect as stereoregularity

The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased.
The glass-transition temperature Tg of a material characterizes the range of temperatures over which this glass transition occurs. It is always lower than the melting temperature, Tm, of the crystalline state of the material, if one exists.
Hard plastics like polystyrene and poly(methyl methacrylate) are used well below their glass transition temperatures, i.e., when they are in their glassy state. Their Tg values are well above room temperature, both at around 100 °C (212 °F).
Factors Affecting Tg
Chemical Structure
- Molecular Weight – In straight chain polymers, increase in MW leads to decrease in chain end concentration resulting in decreases free volume at end group region – and increase in Tg
- Molecular Structure - Insertion of bulky, inflexible side group increases Tg of material due to decrease in mobility,
- Chemical cross-linking - Increase in cross-linking decreases mobility leads to decrease in free volume and increase in Tg
- Polar groups - Presence of polar groups increases intermolecular forces; inter chain attraction and cohesion leading to decrease in free volume resulting in increase in Tg.
Addition of Plasticizers
Addition of plasticizer increases the free volume in polymer structure (Plasticizer gets in between the polymer chains and spaces them apart from each other)This results in polymer chains sliding past each other more easily. As a result, the polymer chains can move around at lower temperatures resulting in decrease in Tg of a polymer
Water or moisture content
Increase in moisture content leads formation of hydrogen bonds with polymeric chains increasing the distance between polymeric chains. And, hence increases the free volume and decreases Tg. Measurement of Tg
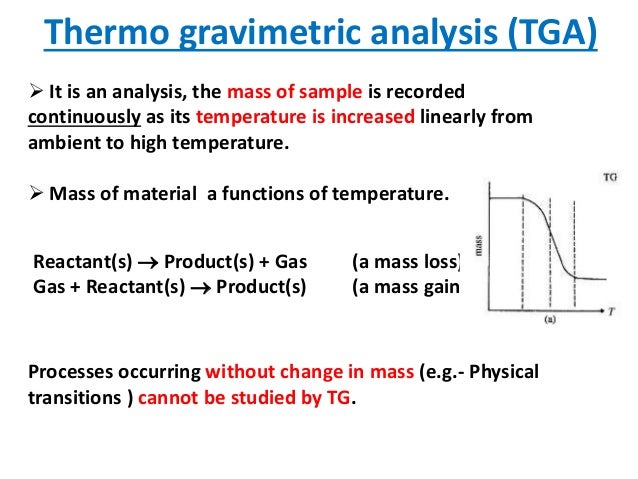
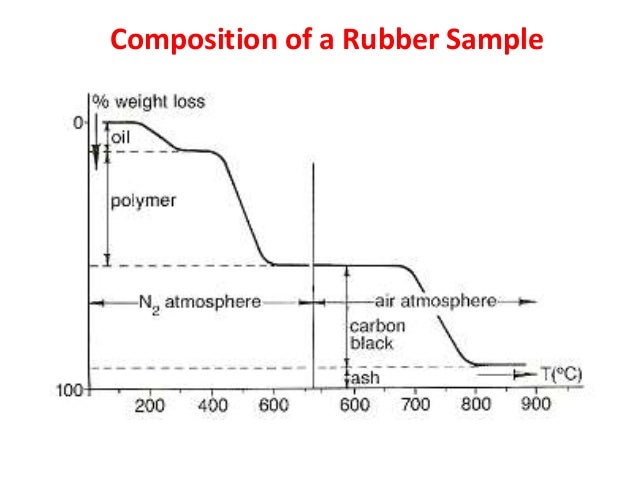
Principle of Differential Scanning Calorimetry (DSC)
DSC is a commercially available instrument which has two (2) types: Heat Flux Type and Power Compensation Type.
Figure 1 shows the block diagram of Heat Flux DSC as an example.
Heat Flux DSC comprises the sample and reference holder, the heat resistor, the heat sink, and the heater.
Heat of heater is supplied into the sample and the reference through heat sink and heat resistor.
Heat flow is proportional to the heat difference of heat sink and holders.
Heat sink has the enough heat capacity compared to the sample.
In case the sample occurs endothermic or exothermic phenomena such as transition and reaction, this endothermic or exothermic phenomena is compensated by heat sink. Thus the temperature difference between the sample and the reference is kept constant.
The difference the amount of heat supplied to the sample and the reference is proportional to the temperature difference of both holders.
By calibrating the standard material, the unknown sample quantitative measurement is achievable.
DSC enables the measurements of the transition such as the glass transition, melting, and crystallization.
Furthermore, the chemical reaction such as thermal curing, heat history, specific heat capacity, and purity analysis are also measurable.
Thermal curing: a chemical process in which a prepolymer is converted into a polymer and then into a network, upon application of heat.
Recently, with the development of the highly-functional polymeric material, these thermal properties analysis needs are increasing dramatically.
(Differential thermal analysis) DTA and DSC detect the temperature differences between the sample and the reference; however, DSC can perform the quantitative measurement of the amount of heat on top.





No comments:
Post a Comment